There is a significant unmet medical need in stage II and III HR+ early breast cancer (eBC) – too many patients remain at risk of recurrence despite current treatment options, underscoring a gap in the treatment landscape.1-4
UNACCEPTABLE RISK
Too many patients with HR+ eBC remain at risk of both early and late recurrence, even after endocrine therapy1-4
Patients with stage II or III disease face a considerable risk of recurrence, regardless of nodal involvement. This risk persists despite adjuvant endocrine therapy and remains a significant concern for decades.1–4
Regardless of nodal status, patients remain at risk of recurrence1,2
Breast cancer recurrence by nodal status within 3 years of starting adjuvant endocrine therapy:

Above are data from control arms of CDK4/6i adjuvant phase III trials in patients with HR+/HER2- eBC.1,2
Metastatic recurrences are a primary concern, because there is currently no cure2,5
Risk within 3 years: by stage and nodal status
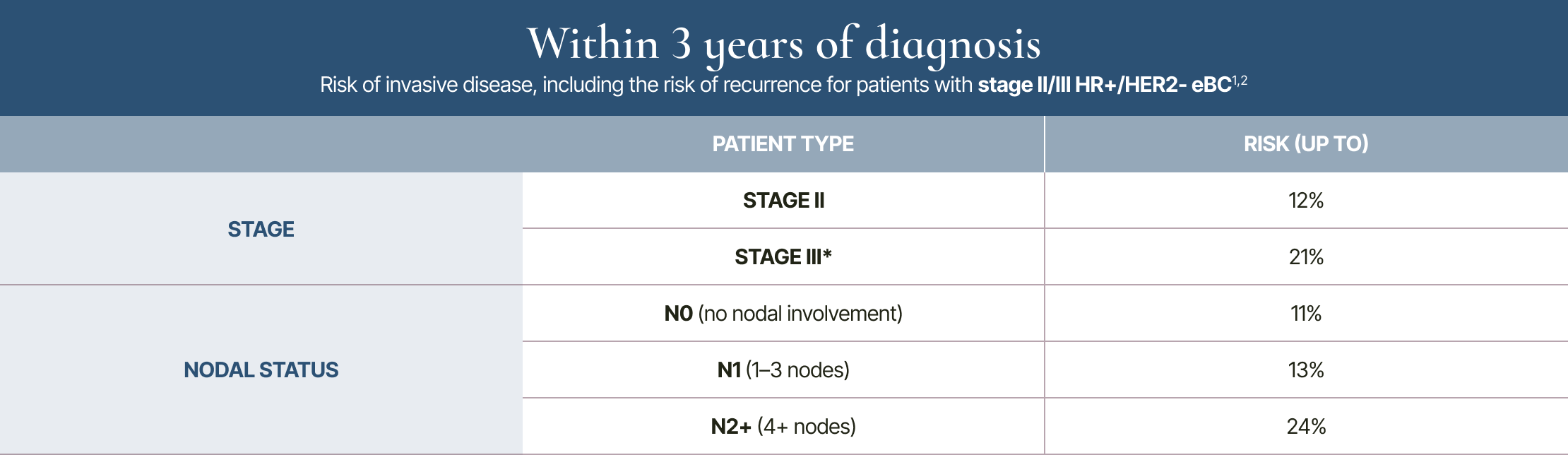
* 3-year rate listed for stage III includes some stage IIB patients, due to differentiated data breakouts between trials.1,2
3-year risk is based on the iDFS outcomes of patients with HR+/HER2- eBC who received endocrine therapy alone in select CDK4/6i clinical trials.1,2 The 3-year and 20-year data are not from a longitudinal study.
Risk within 20 years: by stage and nodal status
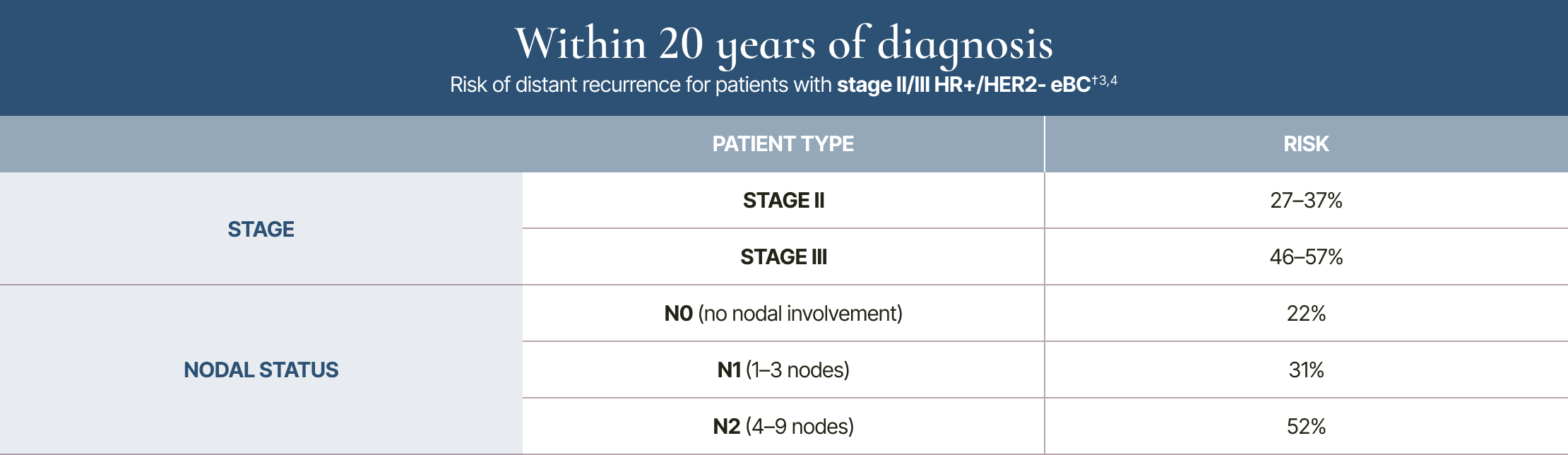
†Analysis included patients with T1/T2 disease and <10 involved nodes.3,4
20-year risk of distant recurrence is from a meta-analysis of 78 randomised trials in the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) database of 74,194 women with HR+ breast cancer who had 5 years of scheduled endocrine therapy.3 The 3-year and 20-year data are not from a longitudinal study.
Assessing risk is complex, requiring consideration of multiple factors6
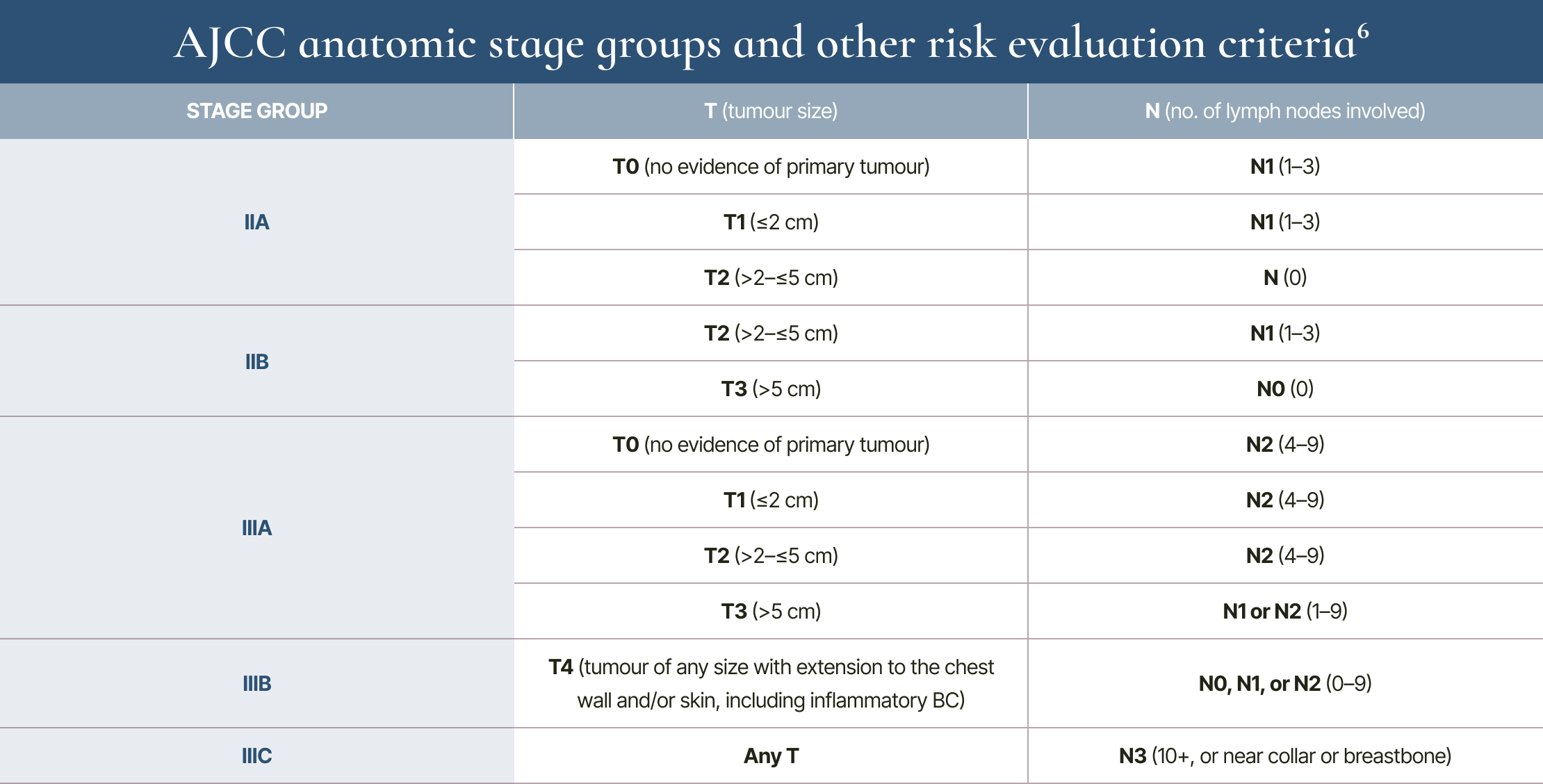
Anatomic stage II and III tumours do not have distant metastases (M0).6
Evaluating risk and prognosis is complex, and many criteria are involved:6
- Tumour size
- Nodal status
- Histologic grade
- ER, PR, and HER2 status
- Genomic profile scores (e.g., Oncotype DX®)
- Circulating tumour cells and disseminated tumour cells
- Ki-67
- Age
- Menopausal status
- Comorbidities
LIMITED OPTIONS
In HR+/HER2- eBC, recently approved adjuvant treatments are limited to patients at the highest risk of recurrence7,8
Adjuvant endocrine therapy has been the standard of care for patients with HR+ eBC for over 3 decades. Though targeted adjuvant treatments have recently been introduced, their limited indications highlight a gap in the treatment landscape.7–12
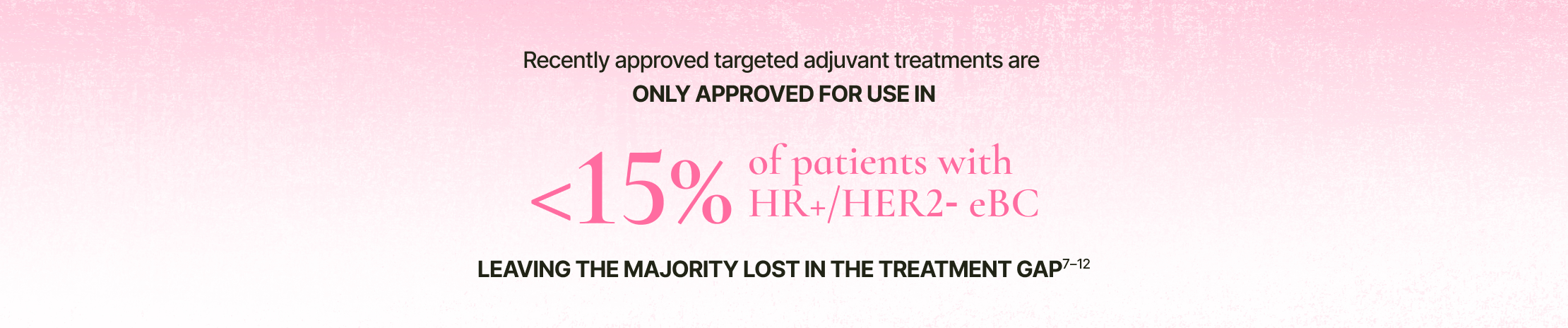
Percentage represents the proportion of eligible patients out of all stages of eBC.
Calculation based on targeted therapy approval for stage II or III node-positive HR+/HER2- disease of BRCA1 and BRCA2 germline mutations in ER+ breast cancer.7–10
TOLERABILITY CHALLENGES
Patients with eBC need treatments that can help them live their fullest lives13–20
It is important that patients can adhere to therapy in order to effectively reduce their risk of recurrence. Many patients will need to be on therapy for years, so their ability to tolerate treatment is paramount. Symptomatic adverse reactions contribute to poor tolerability and are commonly associated with:13–20

CLOSING THE GAP
Closing the gap
Are your patients with stage II or III HR+/HER2- eBC falling into the treatment gap?
Unacceptable risk of recurrence
Patients with stage II or III HR+ eBC remain at risk of recurrence, despite adjuvant endocrine therapy – a risk that persists across their lifetime, regardless of nodal involvement.1–4
Limited treatment options
Recently approved targeted adjuvant treatments are only approved for use in <15% of patients with HR+/HER2- eBC – leaving the majority lost in the treatment gap.7–10
Tolerability challenges
Nonadherence and discontinuation are associated with an increased risk of recurrence. Treatments with improved tolerability are needed.13–15
Committed to closing the gap
For more than 35 years, Novartis has been a key player in the field of breast cancer, focusing on the unmet medical needs of our patients and customers.
Definitions & References
Abbreviations
BC, breast cancer; AJCC, American Joint Committee on Cancer; BRCA, breast cancer gene; CDK4/6i, cyclin-dependent kinase 4 and 6 inhibitor; eBC, early breast cancer; ER, oestrogen receptor; ET, endocrine therapy; HER2-, human epidermal growth factor receptor 2-negative; HR+, hormone receptor-positive; iDFS, invasive disease-free survival; N0, no nodal involvement; N1, 1–3 axillary lymph nodes; N2, 4–9 axillary lymph nodes; PR, progesterone receptor; T1, tumour is 2 cm or less; T2, tumour is more than 2 cm but less than 5 cm.
References:
- Mayer E, et al. Lancet Oncol. 2021;22:212–222.
- Johnston SR, et al. Lancet Oncol. 2023;24:77–90.
- Pan H, et al. N Engl J Med. 2017; 377(19):1836–1846.
- Pan H, et al. N Engl J Med. 2017; 377(19):1836–1846 (supplementary materials).
- Harbeck N, et al. Nat Rev Dis Primers. 2019;5:66.
- Amin MB, et al, eds; American Joint Committee on Cancer. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2017.
- European Medicines Agency. European public assessment reports: Verzenios (abemaciclib). Updated June 17, 2024.Accessed September 2, 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/verzenios
- European Medicines Agency. European public assessment reports: Lynparza (olaparib). Updated August 20, 2024.Accessed September 2, 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/lynparza
- Howlader N, et al. J Natl Cancer Inst. 2014;106(5):dju055.
- Hu C, et al. N Engl J Med. 2021; 384(5):440–451.
- Huppert LA, et al. CA Cancer J Clin. 2023;73(5):480–515.
- Elliott MJ, Cescon DW. Breast. 2022;62(suppl 1):S34–S42.
- Pistilli B, et al. Am Soc Clin Oncol Educ Book. 2022;42:60–72.
- Collin LJ, et al. Clin Cancer Res. 2021;27(5):1421–1428.
- Ejlertsen B, et al. Acta Oncol. 2014;53(2):174–185.
- Ahmed N, et al. JAMA Netw Open. 2022;5(5):e2212327.
- Smith KL, et al. NPJ Breast Cancer. 2022;8(1):53.
- Brett J, et al. Eur J Cancer Care (Engl). 2018;27:1.
- Fontein DBY, et al. Eur J Surg Oncol. 2012;38(2):110–117.
- Hershman DL, et al. J Clin Oncol. 2010;28(27):4120–4128.
This material may include data/information on investigational uses of compounds/drugs that have not yet been approved by regulatory authorities.